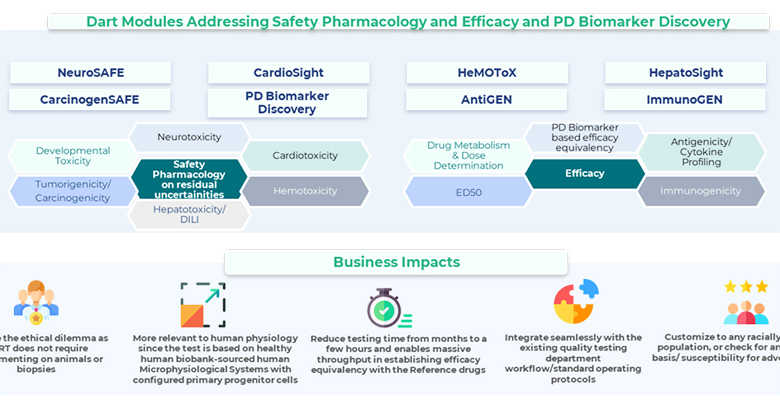
Transcell Oncologics and Quantiphi have together developed an innovative solution known as Digital Animal Replacement Technology (DART), an advancement that harnesses human microphysiological systems and employs Artificial Intelligence (AI) and Machine Learning (ML)-powered digital prediction models. These models are integrated into modular assays that predict safety and efficacy concerns of pharmaceuticals and biopharmaceuticals intended for human use, the companies said in a joint statement today.
[blockquote align=”left” style=”font-size: 30px”]Recently, the United States Food and Drug Administration (USFDA) Modernization Act 2.0 has passed a law to allow alternatives to animal testing [/blockquote]
According to the statement, DART comprises seamless integration into existing workflows, enhancing end-user engagement. This empowers users to assess the safety and efficacy of their assets, providing human-relevant data even before clinical trials commence and sometimes during routine batch testing stages.
Within the developmental cycle of biosimilars, a strategic approach involves a progressive evaluation of biosimilarity and efficacy equivalence. This includes the consideration of conducting animal studies when necessary and appropriate, based on remaining uncertainties. This approach aims to efficiently tailor study requirements, the statement added.
Besides, Hetero Biopharma is also exploring the integration of the DART advanced technology with Transcell and Quantiphi into its operational processes.
“We have started working with Hetero Biopharma’s leadership team in offering some of the non-animal DART residing modules for human cardiotoxicity and immunogenicity like key assessments. We are sure to add value in supporting their routine testing requirements within their processes and workflow – Advantage in adopting DART, which is anti-thesis to contract testing model,” mentioned Dr. S Dravida, Founder and CEO, Transcell Group, representing DART implementation opportunity.
Recently, the United States Food and Drug Administration (USFDA) Modernization Act 2.0 has passed a law to allow alternatives to animal testing.
Dr. Bala Reddy, Director, Hetero Biopharma, said in the statement, “Evaluating the value of animal studies to support regulatory approval of biosimilars is becoming more and more important. In light of guidelines from various regulatory agencies that encourage alternative approaches to animal testing, technologies like Transcell’s human microphysiological systems, in combination with AI & ML-based in-silico modeling, provide opportunities to develop better and more predictive scientific tools to safeguard the environment and the health of both humans and animals.”
DART upholds its vision of sustainable drug discovery and development, while maintaining a steadfast focus on ensuring both safety and efficacy.
Speaking in this regard, Asif Hasan, Co-founder, Quantiphi, Inc, stated in the statement, “DART embodies our unwavering commitment to propel bio/pharmaceutical research and development with the utmost ethical standards. Employing ethically sourced human stem cells, a sophisticated digital workstation, and the prowess of AI, DART forecasts drug safety and efficacy by analysing human microphysiological systems-drug interactions.”